RP903
RP903 is a stable isotope replaced small molecule drug discovered and developed by Risen. It is an inhibitor targeting PI3Kα for advanced malignant solid tumors with PIK3CA mutation. RP903 has applied for patent protection in several countries/regions (CN201910447749.3, US 62/994,378), obtained NMPA and FDA Phase I clinical approvals in May and June 2022, respectively, and is currently in Phase I clinical trials cooperated with TopAlliance, Shanghai.
In pre-clinical studies, RP903 was compared with Alpelisib head-to-head and the results showed that RP903 demonstrated similar efficacy to Alpelisib in human breast cancer cell MCF-7 and BT-474 xenografted tumor mouse models. In addition, RP903 has also shown great efficacy in several animal models of cervical cancer and kidney cancer.
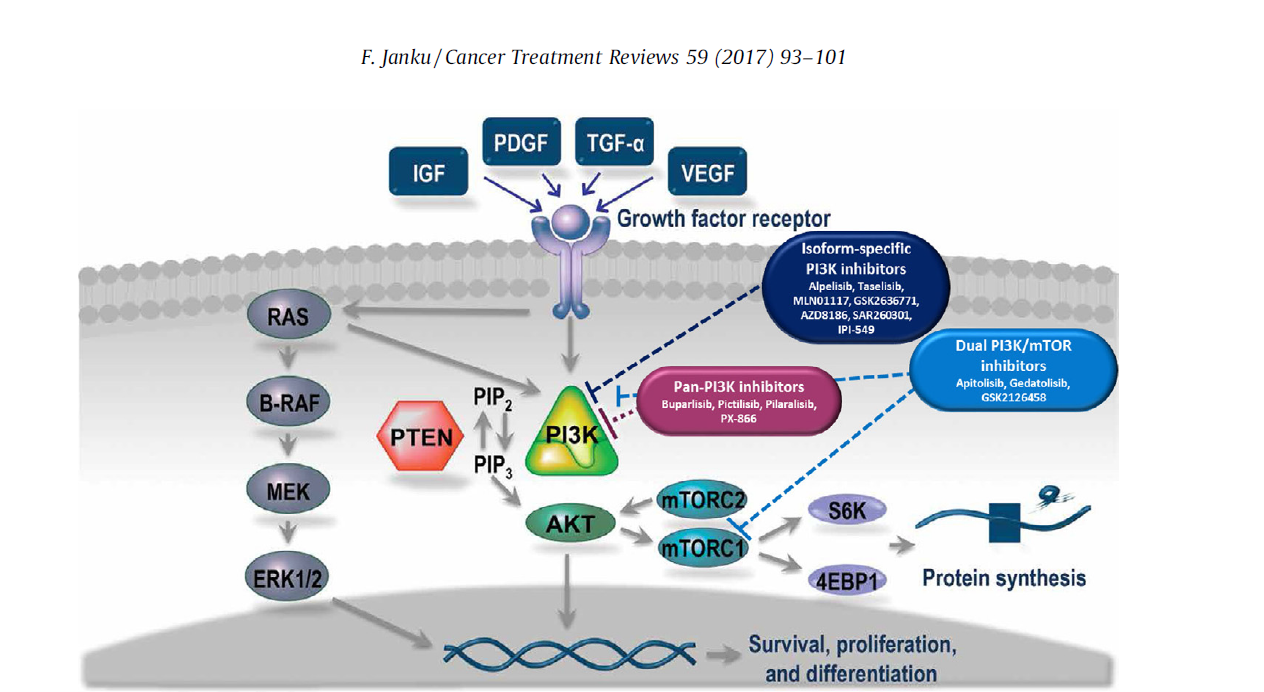
PI3K/Akt/mTOR Pathway

• PAM pathway regulates the survival, proliferation, differentiation and apoptosis of tumor cells
• PI3K in PAM channel can be divided into types Ⅰ, Ⅱ, and Ⅲ
• The mutation of p110α encoded by the PIK3CA gene causes the PI3K kinase to be continuously activated
• This mutation is widespread in malignant solid tumors.